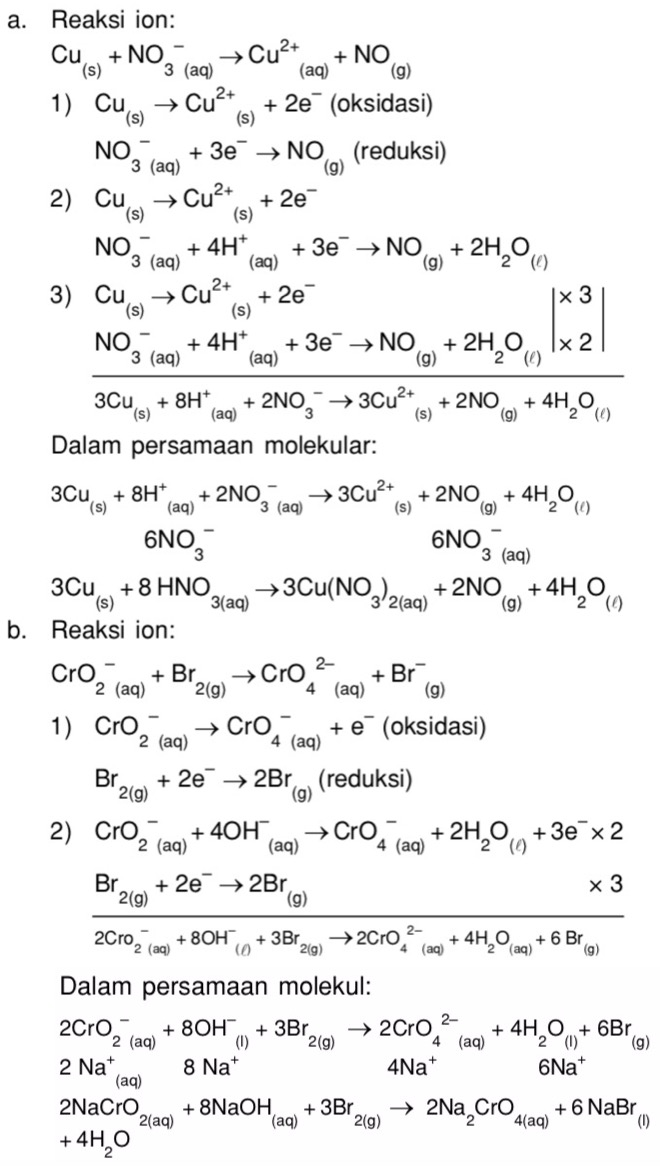
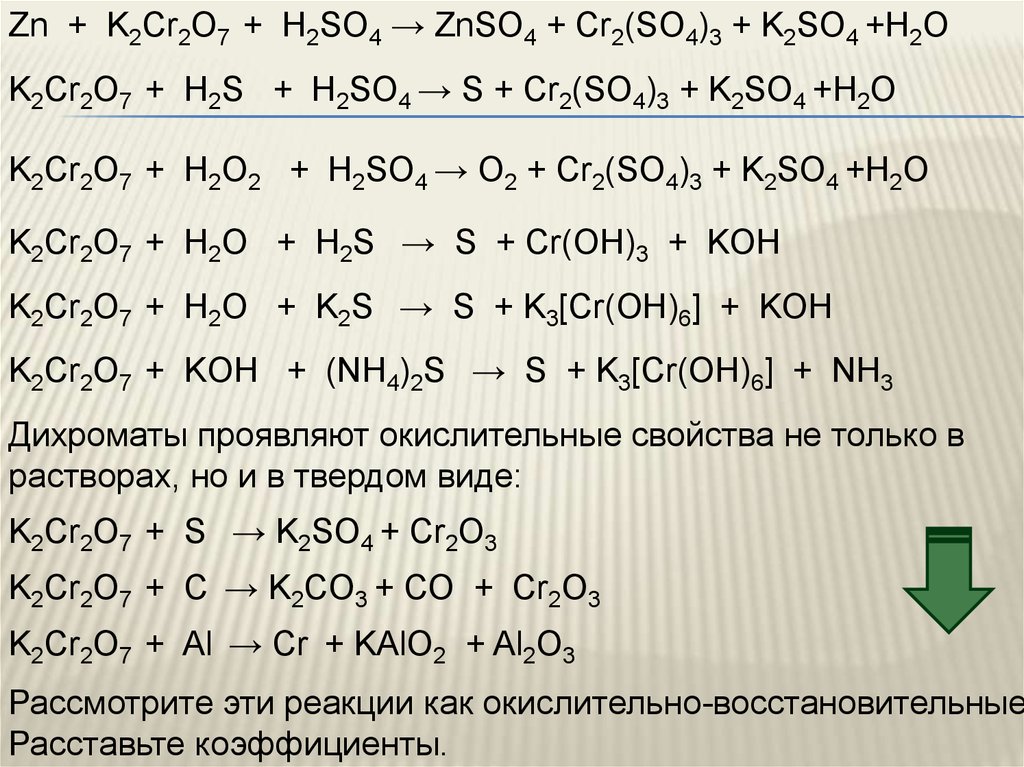
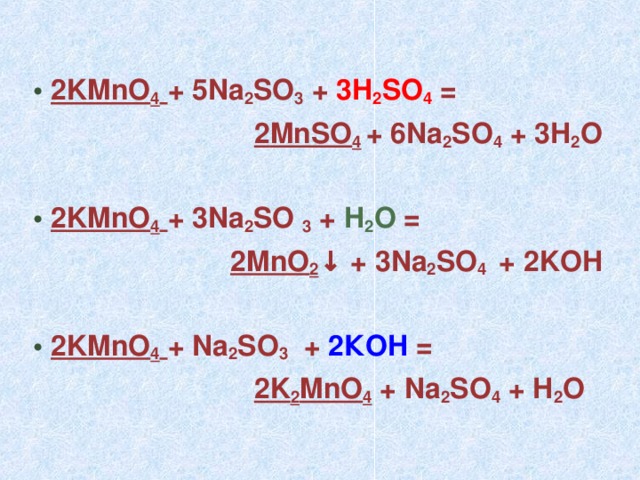
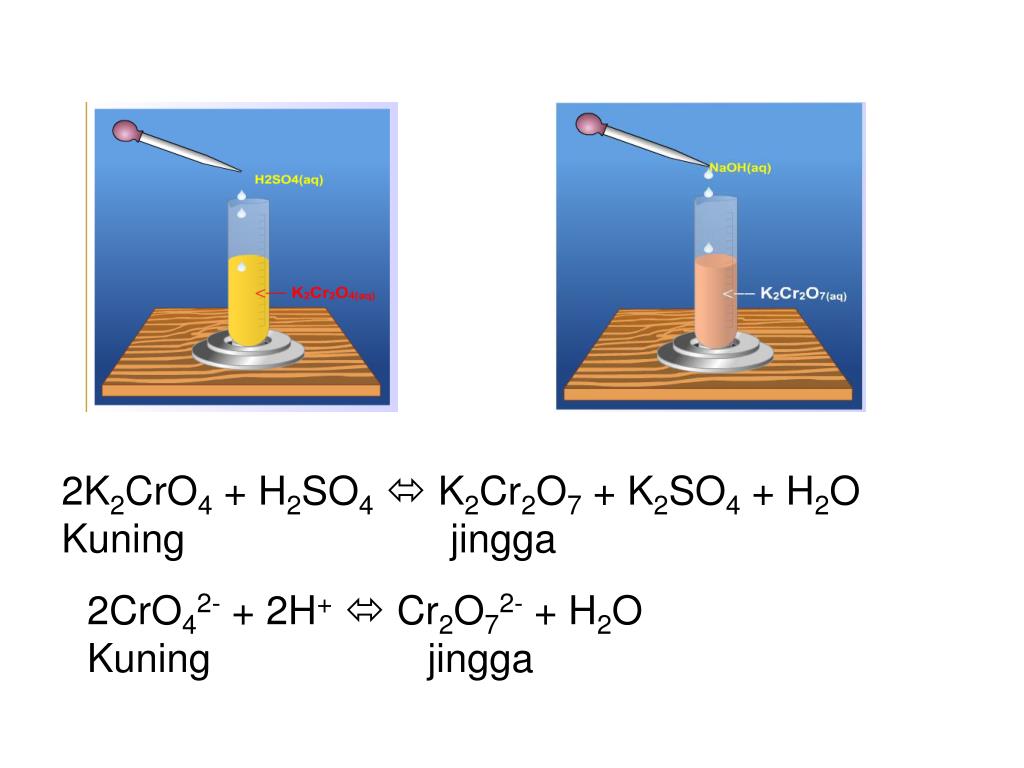
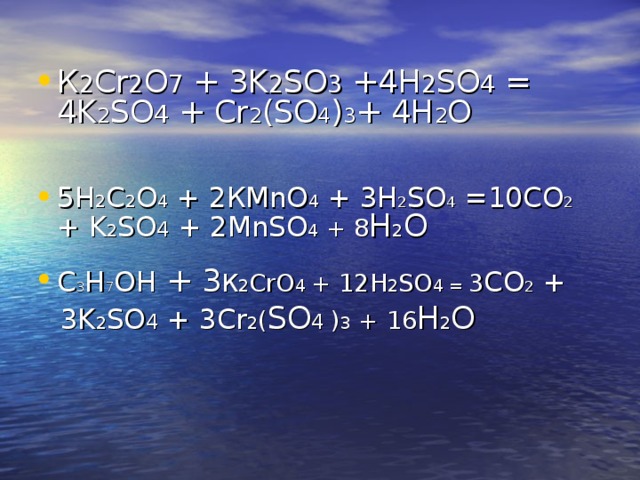Это кислотно-щелочная реакция (нейтрализа́ция): Na2CrO4 является щелочным, H2SO4 представляет собой кислоту.
Это кислотно-щелочная реакция (нейтрализа́ция): Na2CrO4 является щелочным, H2SO4 представляет собой кислоту.
 Запрос NaCrO4 + H2SO4 распознан сайтом как na2cro4+h2so4
Запрос NaCrO4 + H2SO4 распознан сайтом как na2cro4+h2so4
 Solved and balanced chemical equation 2 Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O with completed products.
Solved and balanced chemical equation 2 Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O with completed products.
 It shows the reactants (substances that start a reaction) and products (substances formed by the reaction).
It shows the reactants (substances that start a reaction) and products (substances formed by the reaction).
 Balance the reaction of Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O using this chemical equation balancer!
Balance the reaction of Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O using this chemical equation balancer!
 🚑 Решение задач, контроши, рефераты, курсовые и другое! Онлайн сервис помощи учащимся. Цены в 2-3 раза ниже!
🚑 Решение задач, контроши, рефераты, курсовые и другое! Онлайн сервис помощи учащимся. Цены в 2-3 раза ниже!
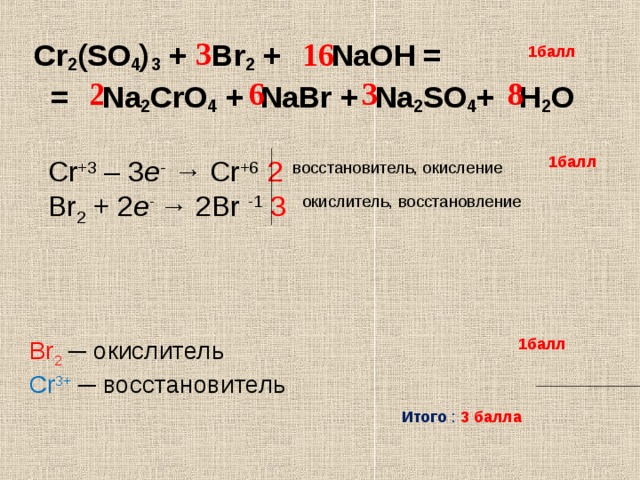
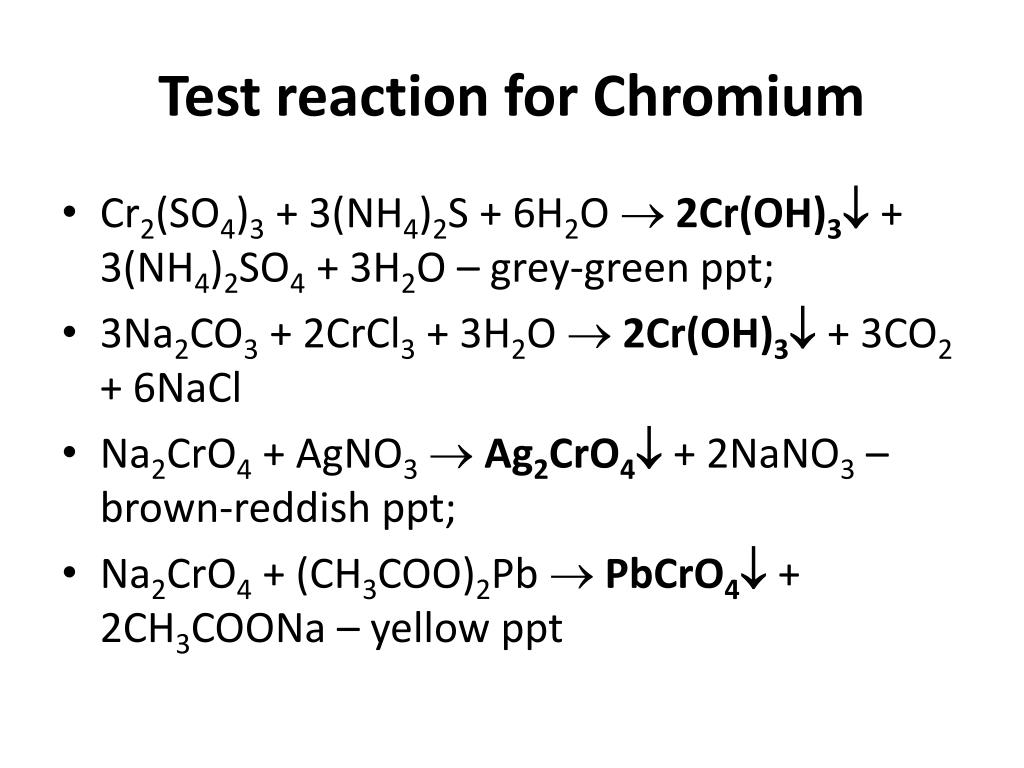
 CrO4 (2-) + 8H (+) + 3e = Cr (3+) + 4H2O 2I (-) - 2e = I2 (0) 2
CrO4 (2-) + 8H (+) + 3e = Cr (3+) + 4H2O 2I (-) - 2e = I2 (0) 2
 Balance the reaction of Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O using this chemical equation balancer!
Balance the reaction of Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O using this chemical equation balancer!
 Cân bằng phương trình hay phản ứng hoá học Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O bằng cách sử dụng máy tính này!
Cân bằng phương trình hay phản ứng hoá học Na2CrO4 + H2SO4 = Na2SO4 + Na2Cr2O7 + H2O bằng cách sử dụng máy tính này!
 Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
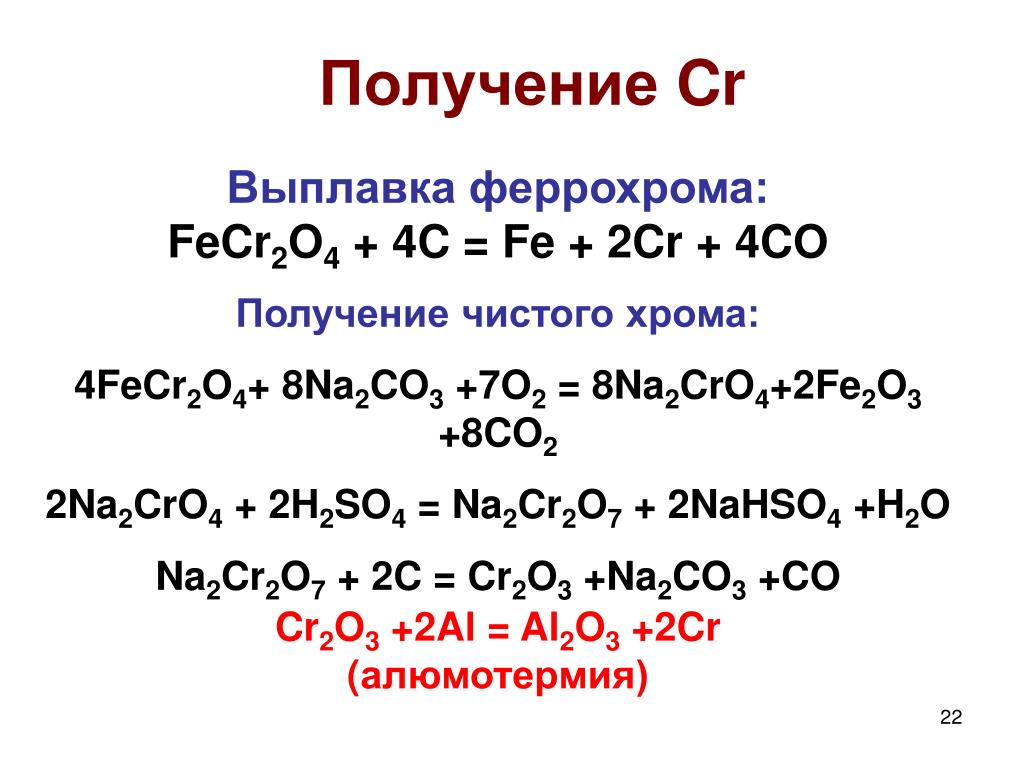
 Кодировка для поиска: 2 Na2CrO4 + H2SO4 = Na2Cr2O7 + Na2SO4 + H2O Ключевые слова: Na2Cr2O7, Na2SO4, h2o, H2SO4, Na2CrO4
Кодировка для поиска: 2 Na2CrO4 + H2SO4 = Na2Cr2O7 + Na2SO4 + H2O Ключевые слова: Na2Cr2O7, Na2SO4, h2o, H2SO4, Na2CrO4
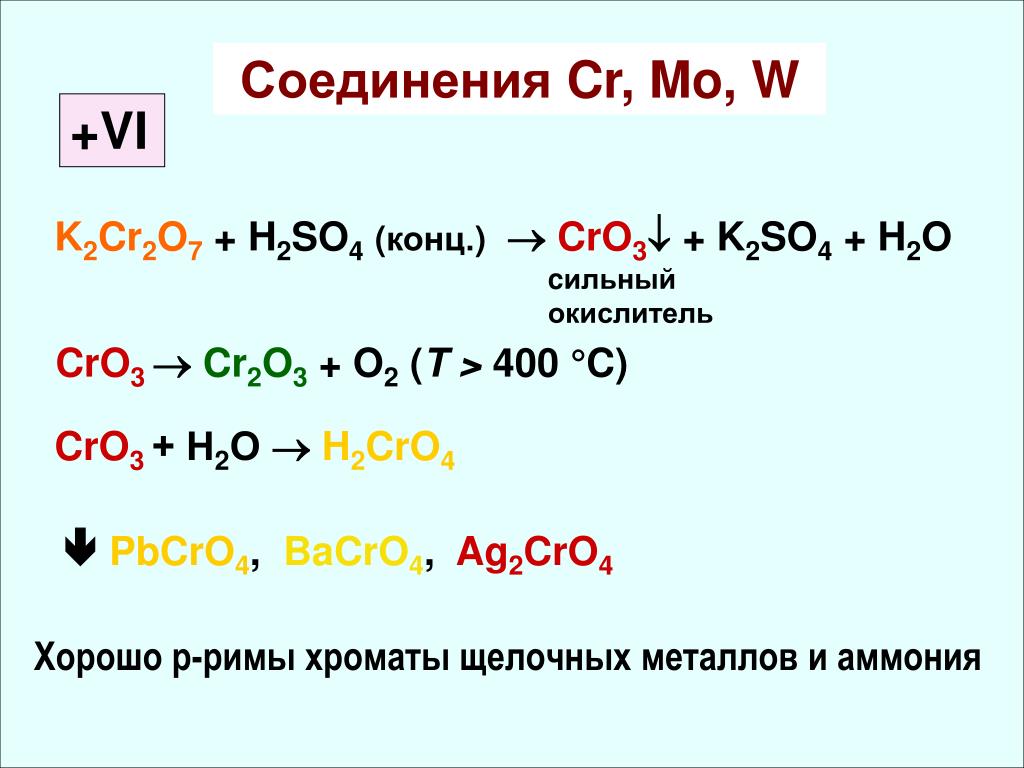
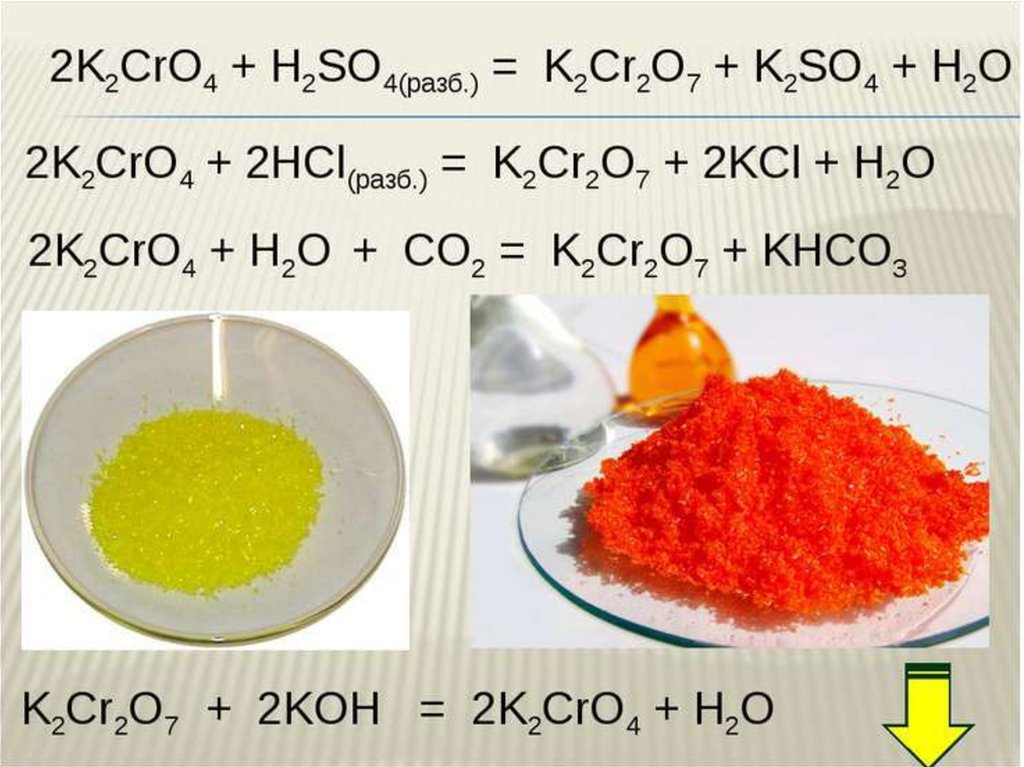
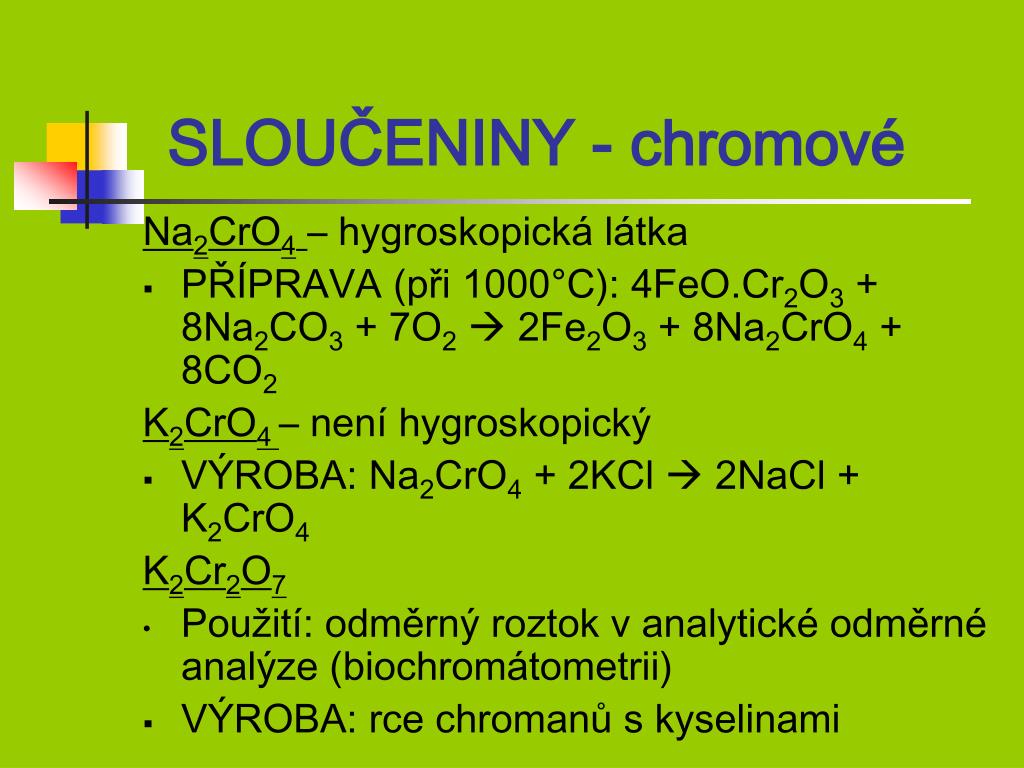
 The key point is that Na 2 CrO 4 (sodium chromate), Na 2 Cr 2 O 7 (sodium dichromate), K 2 CrO 4 (potassium chromate), K 2 Cr 2 O 7 (potassium dichromate), and CrO …
The key point is that Na 2 CrO 4 (sodium chromate), Na 2 Cr 2 O 7 (sodium dichromate), K 2 CrO 4 (potassium chromate), K 2 Cr 2 O 7 (potassium dichromate), and CrO …
 Solved and balanced chemical equation 2 Na2CrO4 + H2SO4 → H2O + Na2SO4 + Na2Cr2O7 with completed products.
Solved and balanced chemical equation 2 Na2CrO4 + H2SO4 → H2O + Na2SO4 + Na2Cr2O7 with completed products.
 So, this SOX2 + NaX2CrOX4 + HX2SOX4 ⟶ NaX2SOX4 + CrX2(SOX4)X3 + HX2O becomes this: SOX2 + CrOX4X − 2 ⟶ SOX4X − 2 + CrX + 3 The sodium ions where …
So, this SOX2 + NaX2CrOX4 + HX2SOX4 ⟶ NaX2SOX4 + CrX2(SOX4)X3 + HX2O becomes this: SOX2 + CrOX4X − 2 ⟶ SOX4X − 2 + CrX + 3 The sodium ions where …
 The reaction of sodium chromate (NaCrO4) and sulfuric acid (H2SO4) May be represented by the following chemical equation2 NaCrO4 + H2SO4 —> Na2Cr2O7+ Na2SO4 + H2O determine …
The reaction of sodium chromate (NaCrO4) and sulfuric acid (H2SO4) May be represented by the following chemical equation2 NaCrO4 + H2SO4 —> Na2Cr2O7+ Na2SO4 + H2O determine …
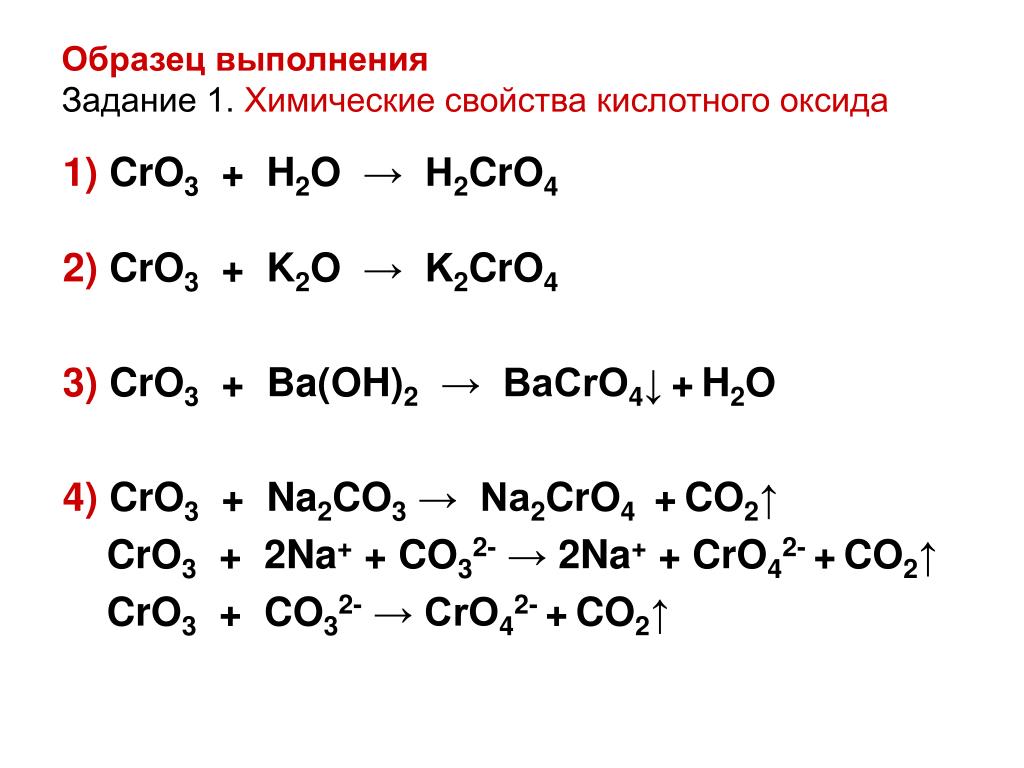
 Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Balancing step by step using the inspection method; Let's balance this equation using the inspection method. First, we set all coefficients to 1:
Еще по теме:



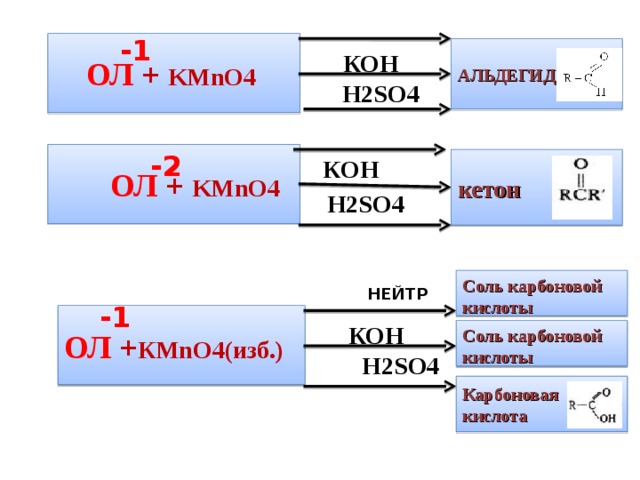





Еще по теме: