Это окислительно-восстановительная (редокс) реакция: MgS является восстановителем, MgS является окислителем, O2 является окислителем. Решенное и коэффициентами …
Это окислительно-восстановительная (редокс) реакция: MgS является восстановителем, MgS является окислителем, O2 является окислителем. Решенное и коэффициентами …
 MgS + O2 = MgO + SO2 is a Double Displacement (Metathesis) reaction where two moles of Magnesium Sulfide [MgS] and three moles of Dioxygen [O 2] react to form two moles of …
MgS + O2 = MgO + SO2 is a Double Displacement (Metathesis) reaction where two moles of Magnesium Sulfide [MgS] and three moles of Dioxygen [O 2] react to form two moles of …
 For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: H 2 + O 2 = H 2 O However, this equation isn't balanced because the number of …
For example, in the reaction of hydrogen (H₂) with oxygen (O₂) to form water (H₂O), the chemical equation is: H 2 + O 2 = H 2 O However, this equation isn't balanced because the number of …
 1 MgS + 1 O 2 = 1 MgO + 1 S 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. S is …
1 MgS + 1 O 2 = 1 MgO + 1 S 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in products. S is …
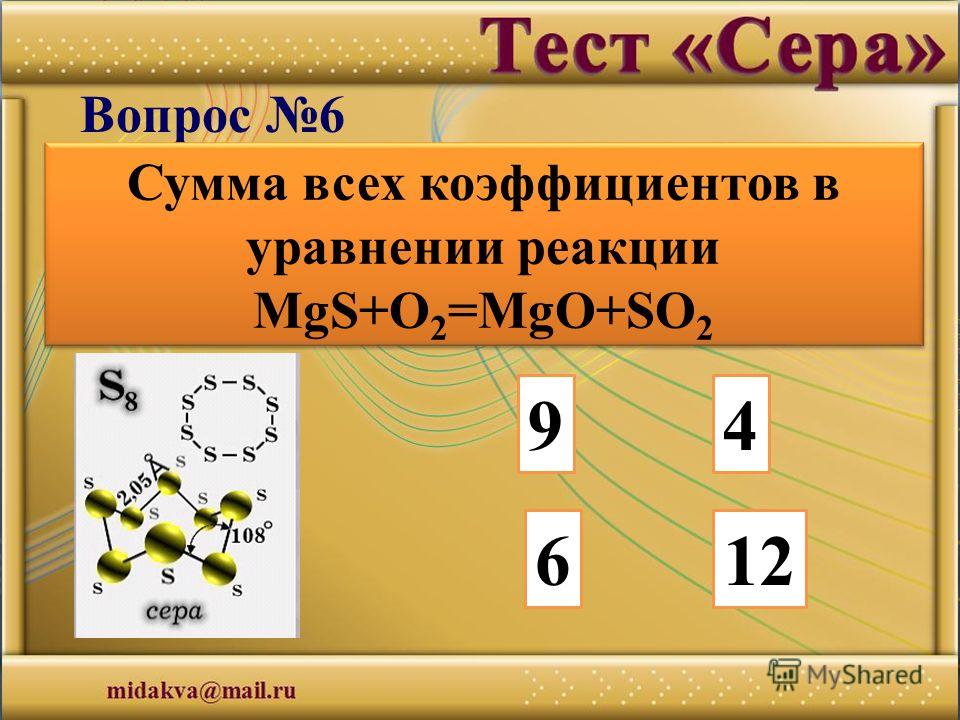
 Решенное и коэффициентами уравнение реакции 3 O2 + 2 MgS → 2 SO2 + 2 MgO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции 3 O2 + 2 MgS → 2 SO2 + 2 MgO с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
 MgS + O2 = MgO + S is a Single Displacement (Substitution) reaction where two moles of Magnesium Sulfide [MgS] and one mole of Dioxygen [O 2] react to form two moles of …
MgS + O2 = MgO + S is a Single Displacement (Substitution) reaction where two moles of Magnesium Sulfide [MgS] and one mole of Dioxygen [O 2] react to form two moles of …
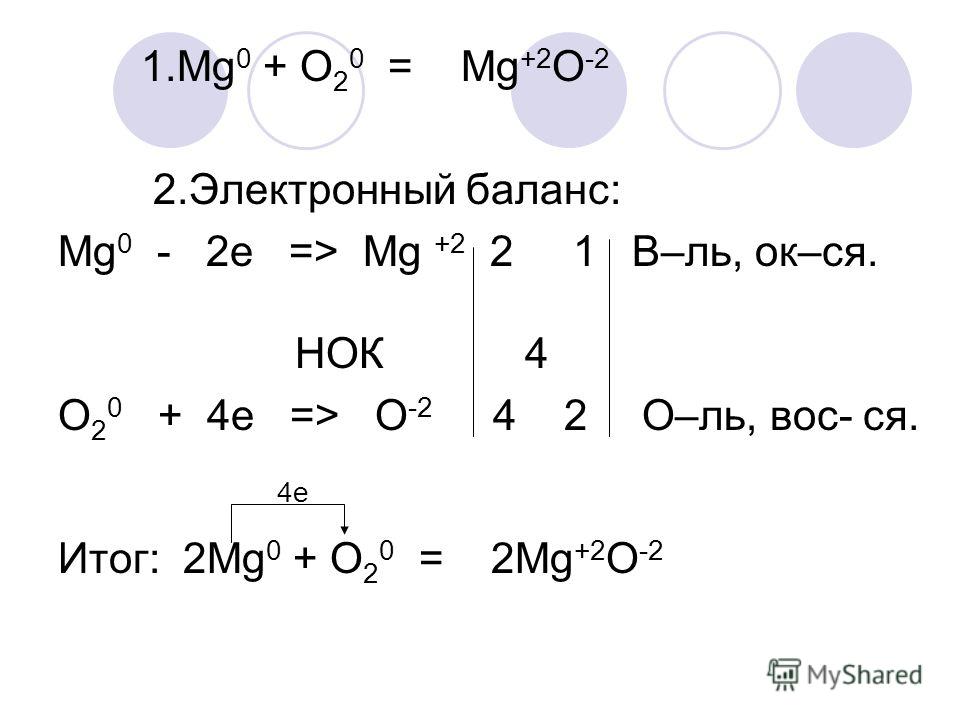
 Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The burning of magnesium metal reacts with oxygen found in the air (oxygen gas) to form magnesium oxide. …
Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The burning of magnesium metal reacts with oxygen found in the air (oxygen gas) to form magnesium oxide. …
 Mg + O2S = MgS + O2 is a Single Displacement (Substitution) reaction where one mole of Magnesium [Mg] and one mole of Sulfur Dioxide [O 2 S] react to form one mole of Magnesium …
Mg + O2S = MgS + O2 is a Single Displacement (Substitution) reaction where one mole of Magnesium [Mg] and one mole of Sulfur Dioxide [O 2 S] react to form one mole of Magnesium …
 Химическая реакция. MgS+O2 = ? MgO+SO2 или Mg+SO2 Roman Профи (523), закрыт 11 лет назад . Лучший.
Химическая реакция. MgS+O2 = ? MgO+SO2 или Mg+SO2 Roman Профи (523), закрыт 11 лет назад . Лучший.
 Units: molar mass - g/mol, weight - g. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the equation. Mg …
Units: molar mass - g/mol, weight - g. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the equation. Mg …
 Magnesium sulfide is an inorganic compound with the formula Mg S. It is a white crystalline material but often is encountered in an impure form that is brown and non-crystalline powder. It is generated industrially in the production of metallic …
Magnesium sulfide is an inorganic compound with the formula Mg S. It is a white crystalline material but often is encountered in an impure form that is brown and non-crystalline powder. It is generated industrially in the production of metallic …
 Pergunta Balancear as equações abaixo pelo método das tentativas: a) SO2 + O2 → SO3 b) N2 + H2 → NH3 c) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O d) Mg + H3PO4 → …
Pergunta Balancear as equações abaixo pelo método das tentativas: a) SO2 + O2 → SO3 b) N2 + H2 → NH3 c) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O d) Mg + H3PO4 → …
 MgS + O2 = Mg + O2S might be a redox reaction. Thermodynamics of the reaction can be calculated using a lookup table. Use the calculator below to balance chemical equations and …
MgS + O2 = Mg + O2S might be a redox reaction. Thermodynamics of the reaction can be calculated using a lookup table. Use the calculator below to balance chemical equations and …
 Mg + O2 = MgO is a Synthesis reaction where two moles of Magnesium [Mg] and one mole of Dioxygen [O 2] combine to form two moles of Magnesium Oxide [MgO]
Mg + O2 = MgO is a Synthesis reaction where two moles of Magnesium [Mg] and one mole of Dioxygen [O 2] combine to form two moles of Magnesium Oxide [MgO]
 1 MgS(s) + 1 O 2 (g) = 1 MgO(s) + 1 SO 2 (g) For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in …
1 MgS(s) + 1 O 2 (g) = 1 MgO(s) + 1 SO 2 (g) For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is balanced: 1 atom in reagents and 1 atom in …
 Balance the reaction of MgS + O2 = MgS + O2 using this chemical equation balancer!
Balance the reaction of MgS + O2 = MgS + O2 using this chemical equation balancer!
Еще по теме:
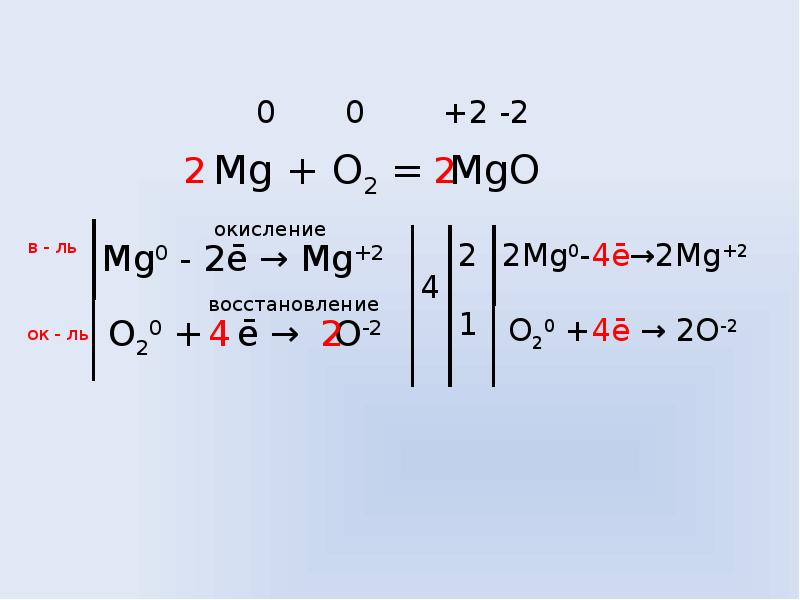













Еще по теме: