Решенное и коэффициентами уравнение реакции Fe2O3 + 2 Al → 2 Fe + Al2O3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.
Решенное и коэффициентами уравнение реакции Fe2O3 + 2 Al → 2 Fe + Al2O3 с дополненными продуктами. Приложение для вычисления и дополнения продуктов реакции.

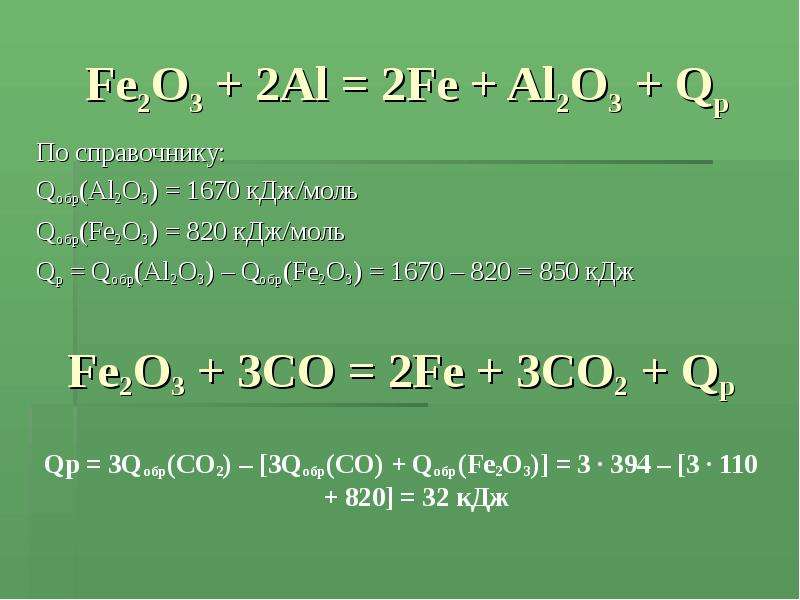 Fe2O3 + Al = Fe + Al2O3 is a Single Displacement (Substitution) reaction where one mole of Hematite [Fe 2 O 3] and two moles of Aluminium [Al] react to form two moles of Iron [Fe] and …
Fe2O3 + Al = Fe + Al2O3 is a Single Displacement (Substitution) reaction where one mole of Hematite [Fe 2 O 3] and two moles of Aluminium [Al] react to form two moles of Iron [Fe] and …
 Solution. The correct option is D. Displacement reaction. An explanation for Correct answer:- Option (D) Displacement reaction. A displacement reaction is one wherein the atom or a set of …
Solution. The correct option is D. Displacement reaction. An explanation for Correct answer:- Option (D) Displacement reaction. A displacement reaction is one wherein the atom or a set of …
 Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, …
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, …
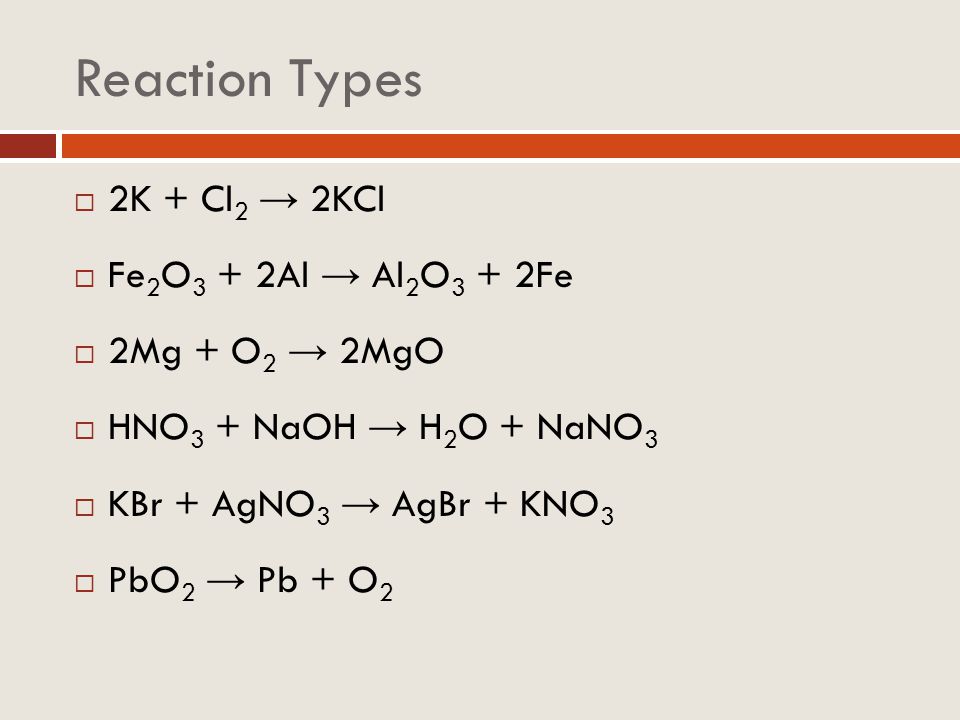
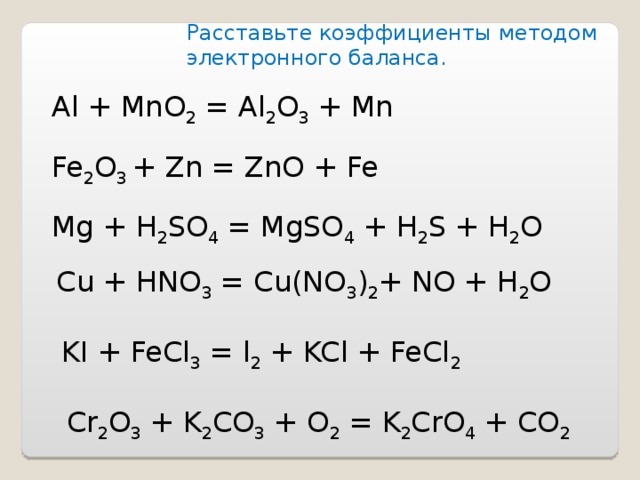
 Характеристика химической реакции. Образование оксида алюминия и железа из оксида железа (III) и алюминия. Молекулярное уравнение. Fe 2 O 3 + 2Al = Al 2 O 3 + 2Fe. …
Характеристика химической реакции. Образование оксида алюминия и железа из оксида железа (III) и алюминия. Молекулярное уравнение. Fe 2 O 3 + 2Al = Al 2 O 3 + 2Fe. …
 Explanation: The coefficients in the equation provide a molar ratio between the chemical species in the equation, so you must first convert the mass of iron (III) oxide to moles …
Explanation: The coefficients in the equation provide a molar ratio between the chemical species in the equation, so you must first convert the mass of iron (III) oxide to moles …
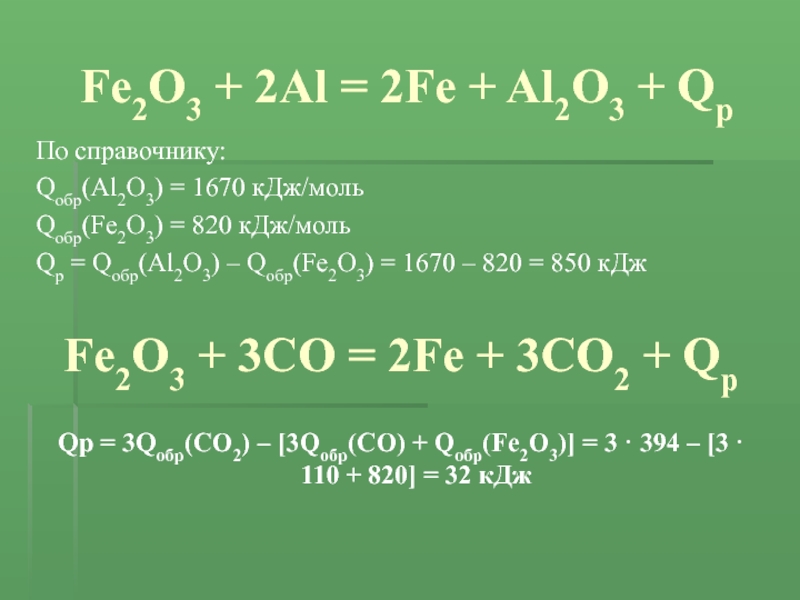 Al + Fe2O3 = Al2O3 + Fe is a Single Displacement (Substitution) reaction where two moles of Aluminium [Al] and one mole of Hematite [Fe 2 O 3] react to form one mole of Aluminum Oxide …
Al + Fe2O3 = Al2O3 + Fe is a Single Displacement (Substitution) reaction where two moles of Aluminium [Al] and one mole of Hematite [Fe 2 O 3] react to form one mole of Aluminum Oxide …
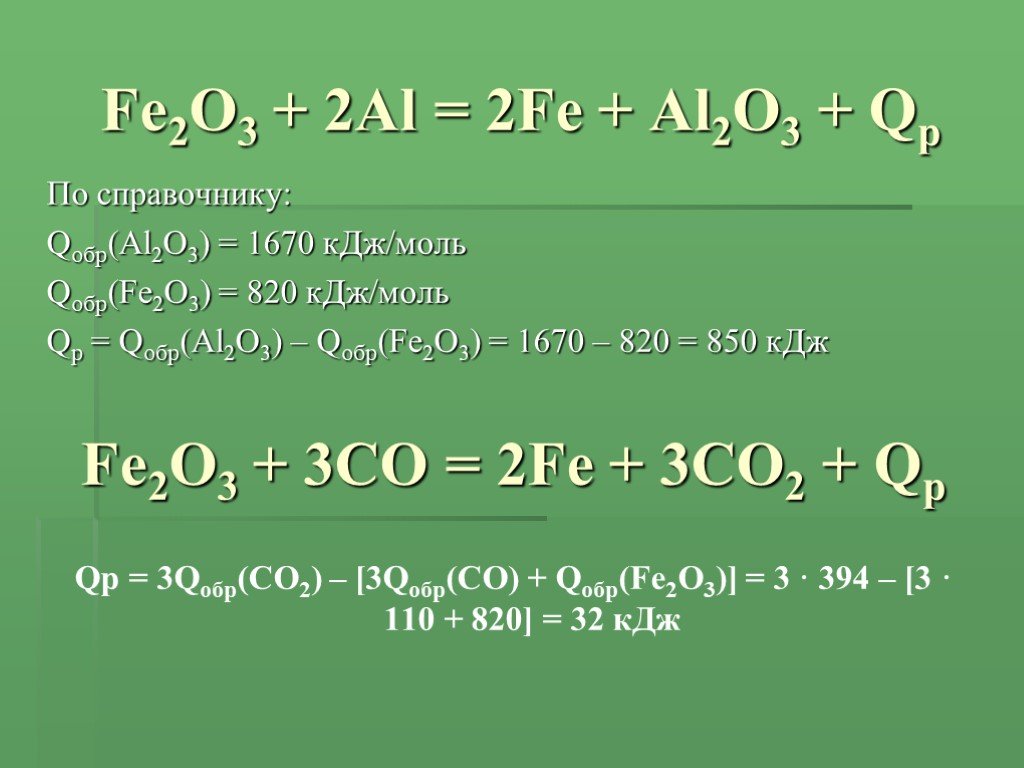 Examine this equation and answer the questions that follow: 2Al + Fe2O3 → Al2O3 + 2Fe How many grams of Fe can be produced when 10.0g of Al is reacted with an …
Examine this equation and answer the questions that follow: 2Al + Fe2O3 → Al2O3 + 2Fe How many grams of Fe can be produced when 10.0g of Al is reacted with an …
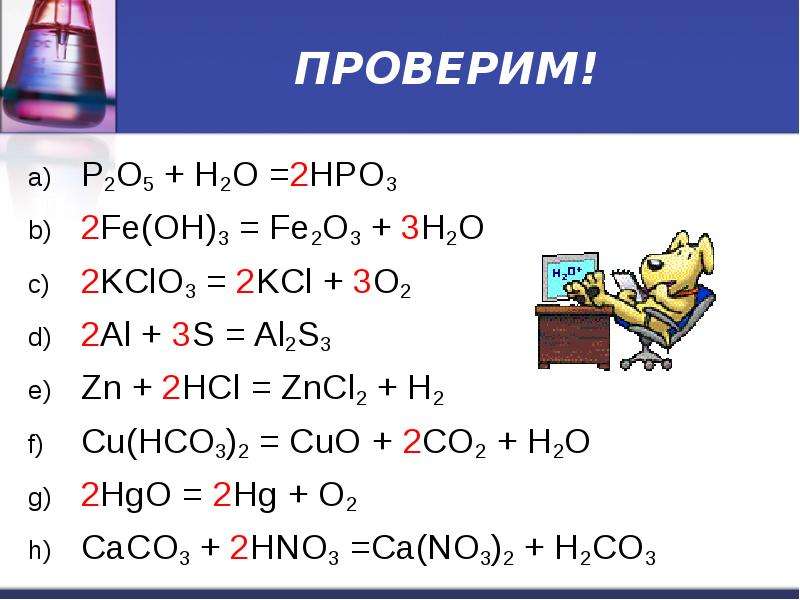
 Уравнение Fe2O3 + 2Al = 2Fe + Al2O3 - Q тип реакции: а)эндотермическая реакция обмена. б)экзотермиче - ответ на этот и другие вопросы получите онлайн на сайте Uchi.ru.
Уравнение Fe2O3 + 2Al = 2Fe + Al2O3 - Q тип реакции: а)эндотермическая реакция обмена. б)экзотермиче - ответ на этот и другие вопросы получите онлайн на сайте Uchi.ru.
 Solution. Redox Reaction. Oxidation is a process that involves the addition of oxygen or any electronegative element or the removal or an electropositive element. Reduction is a process …
Solution. Redox Reaction. Oxidation is a process that involves the addition of oxygen or any electronegative element or the removal or an electropositive element. Reduction is a process …
 Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Al + 1 Fe 2 O 3 = 1 Al 2 O 3 + 1 Fe. For each element, we check if the number of atoms is balanced …
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Al + 1 Fe 2 O 3 = 1 Al 2 O 3 + 1 Fe. For each element, we check if the number of atoms is balanced …
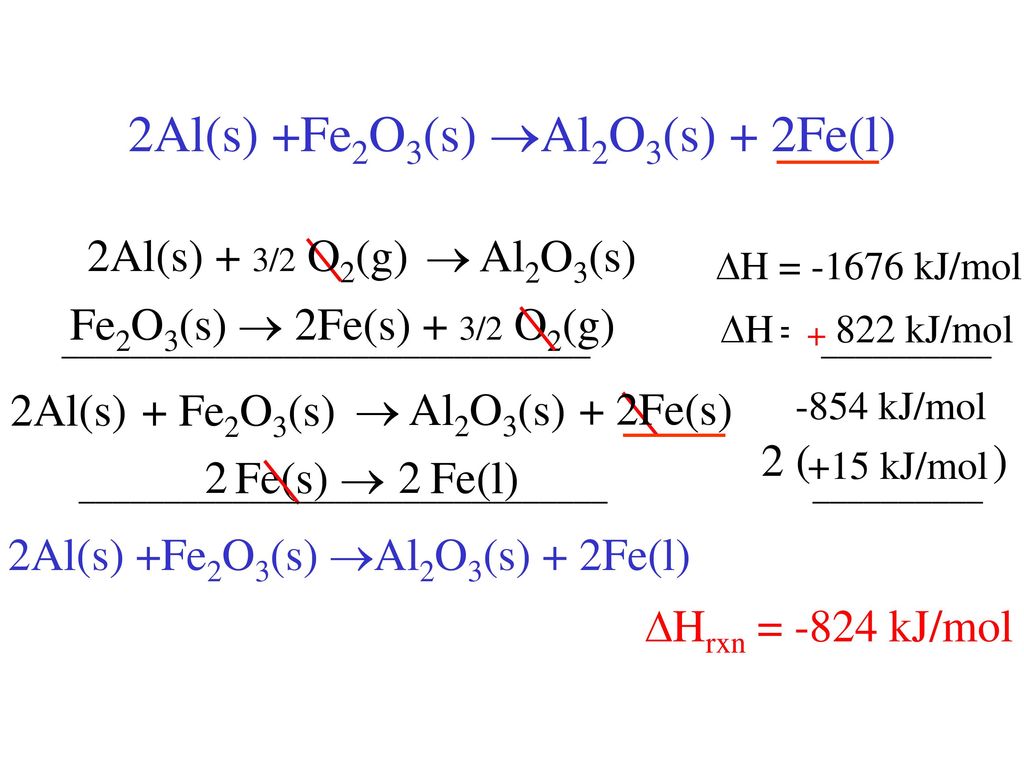 4) Записываем уравнение в молекулярном виде: Fe2O3 + 2Al = Al2O3 + 2Fe Небольшая поправка к ответу Виктора: если знак ("+" или "-") стоит перед символом элемента - то …
4) Записываем уравнение в молекулярном виде: Fe2O3 + 2Al = Al2O3 + 2Fe Небольшая поправка к ответу Виктора: если знак ("+" или "-") стоит перед символом элемента - то …
 In this video we will balance the equation Al + Fe2O3 = Al2O3 + Fe . Visit https://www.Breslyn.org for video guides on balancing equations and more!To balan.
In this video we will balance the equation Al + Fe2O3 = Al2O3 + Fe . Visit https://www.Breslyn.org for video guides on balancing equations and more!To balan.
 2Al + Fe 2 O 3 → Al 2 O 3 + 2Fe. How many grams of Fe can be produced when 48.0g of Al is reacted with an excess supply of Fe 2 O 3? There are 2 steps to solve this one. Share Share.
2Al + Fe 2 O 3 → Al 2 O 3 + 2Fe. How many grams of Fe can be produced when 48.0g of Al is reacted with an excess supply of Fe 2 O 3? There are 2 steps to solve this one. Share Share.
 Explanation: One must first balance the metals before balancing non-metals such as Oxygen and Hydrogen (Hydrogen isn't involved in this case). So therefore on the right hand …
Explanation: One must first balance the metals before balancing non-metals such as Oxygen and Hydrogen (Hydrogen isn't involved in this case). So therefore on the right hand …
Еще по теме:









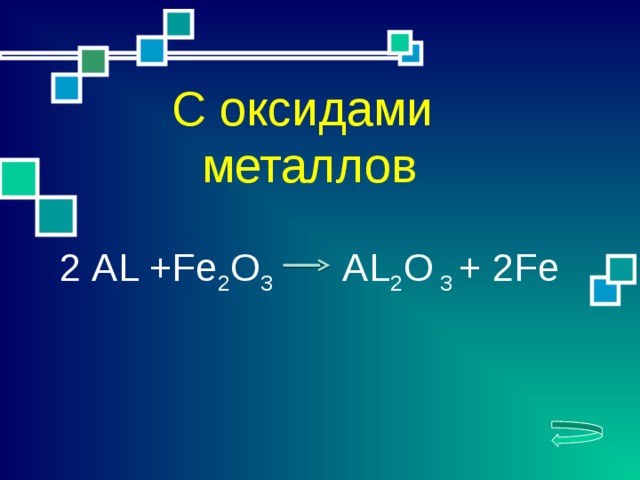




Еще по теме: