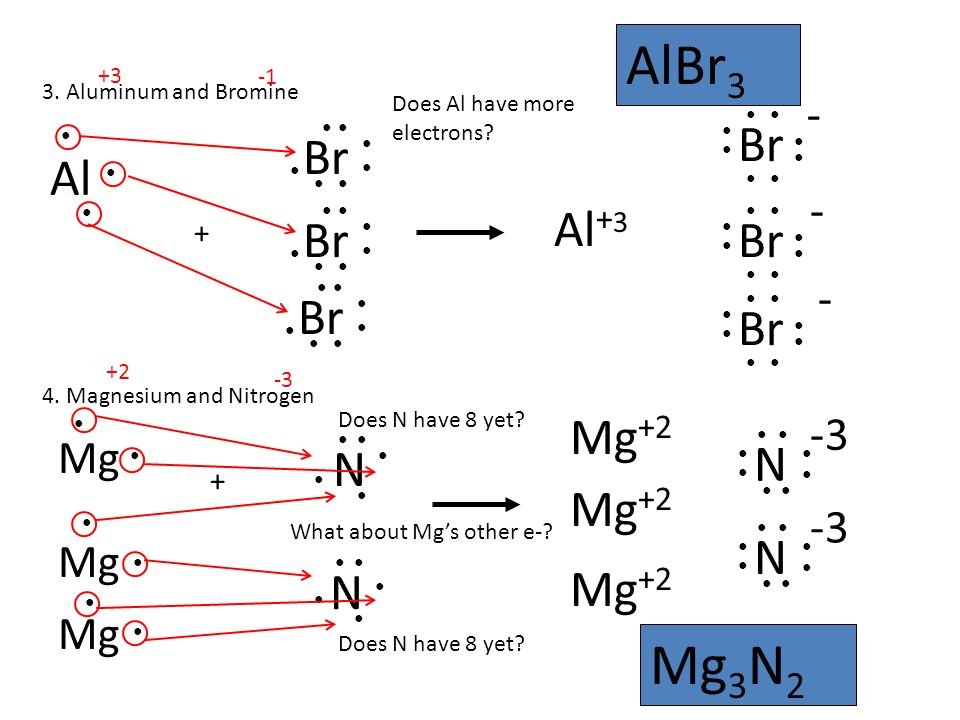
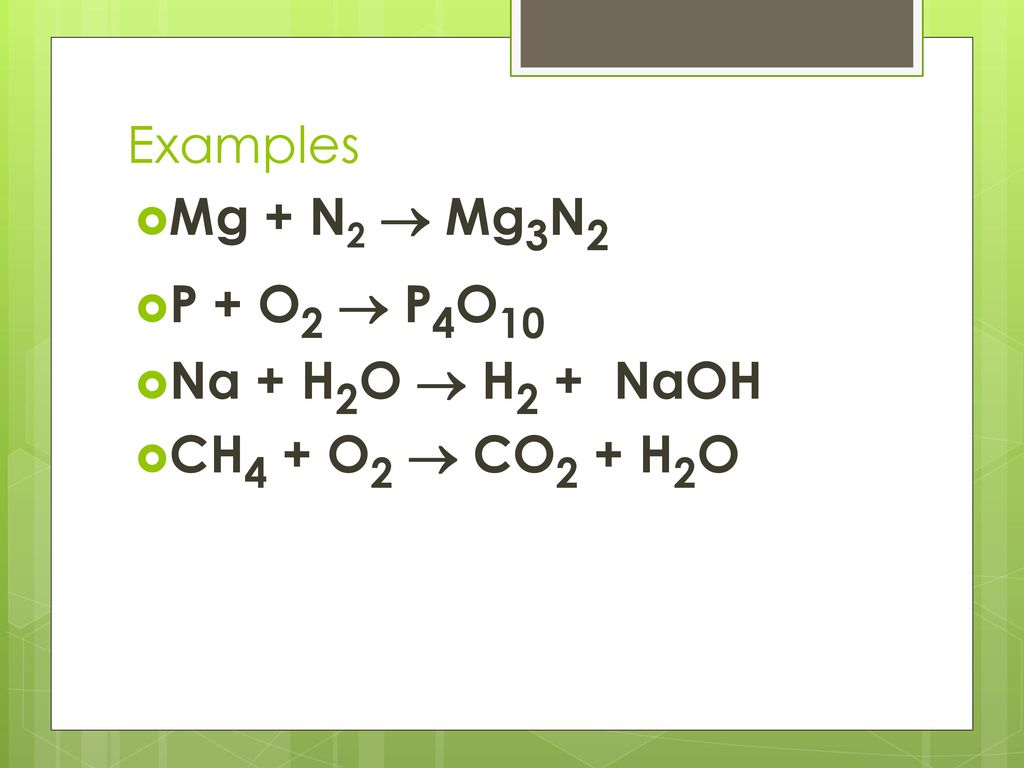
Mg является восстановителем, N2 является окислителем. Решенное и коэффициентами уравнение реакции 3 Mg + N2 → Mg3N2 с дополненными продуктами. Приложение для …
Mg является восстановителем, N2 является окислителем. Решенное и коэффициентами уравнение реакции 3 Mg + N2 → Mg3N2 с дополненными продуктами. Приложение для …
 Solved and balanced chemical equation 3 Mg + N2 → Mg3N2 with completed products. Application for completing products and balancing equations.
Solved and balanced chemical equation 3 Mg + N2 → Mg3N2 with completed products. Application for completing products and balancing equations.
 1 Mg + 1 N 2 = 1 Mg 3 N 2 * Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Mg не сбалансирован: 1 атомов в …
1 Mg + 1 N 2 = 1 Mg 3 N 2 * Для каждого элемента мы проверяем, сбалансировано ли количество атомов в обеих частях уравнения. Mg не сбалансирован: 1 атомов в …
 Mg + N2 = Mg3N2 is a Synthesis reaction where three moles of Magnesium [Mg] and one mole of Dinitrogen [N 2] combine to form one mole of Magnesium Nitride [Mg 3 N 2]
Mg + N2 = Mg3N2 is a Synthesis reaction where three moles of Magnesium [Mg] and one mole of Dinitrogen [N 2] combine to form one mole of Magnesium Nitride [Mg 3 N 2]
 1 Mg + 1 N 2 = 1 Mg 3 N 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is not balanced: 1 atom in reagents and 3 atoms in products.
1 Mg + 1 N 2 = 1 Mg 3 N 2 For each element, we check if the number of atoms is balanced on both sides of the equation. Mg is not balanced: 1 atom in reagents and 3 atoms in products.
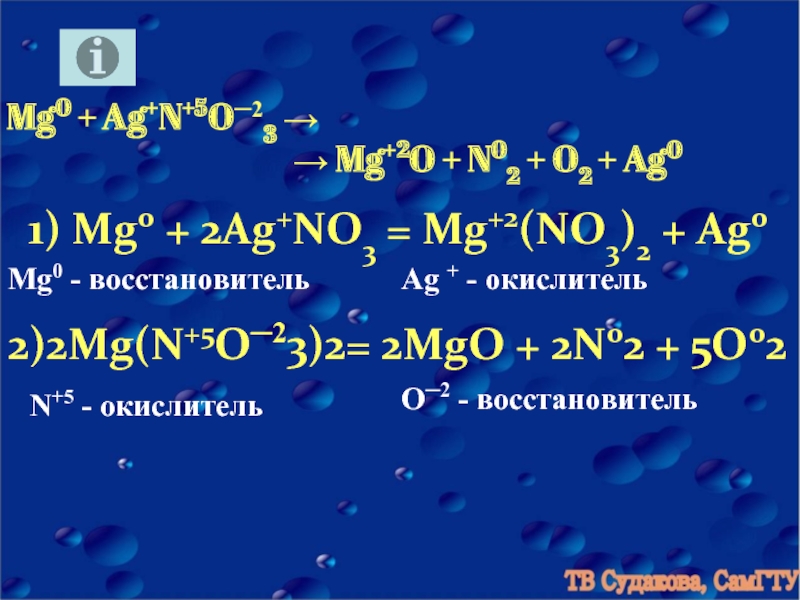
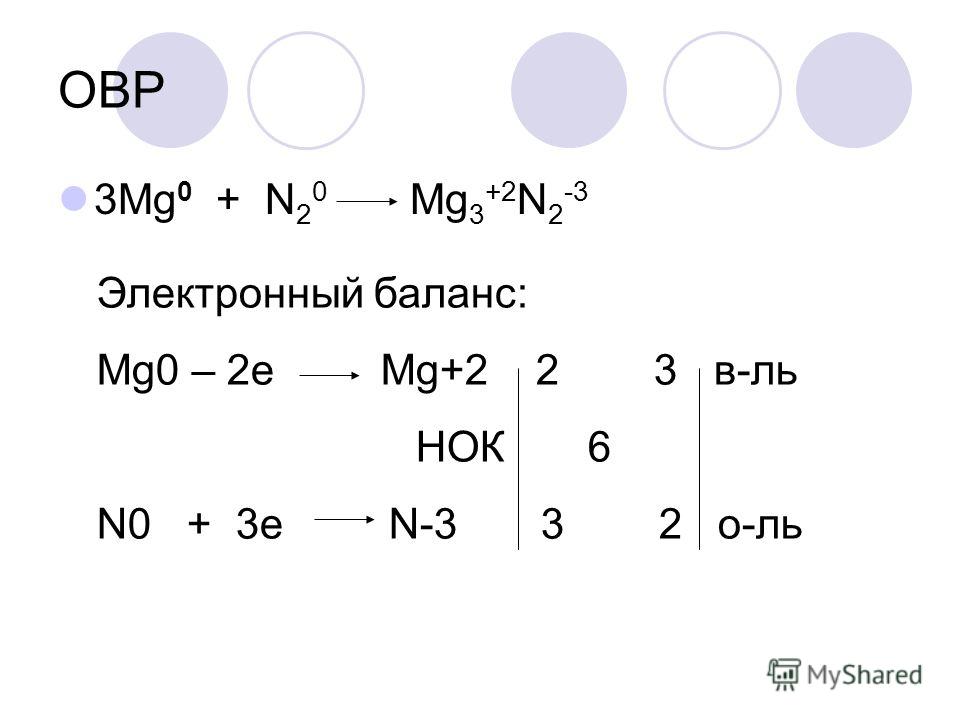
 3Mg + N2 = Mg3N2 is a redox reaction where Mg is oxidized and N is reduced. Mg is a reducing agent (i.e. it lost electrons) and N 2 is a oxidizing agent (i.e. it gained electrons).
3Mg + N2 = Mg3N2 is a redox reaction where Mg is oxidized and N is reduced. Mg is a reducing agent (i.e. it lost electrons) and N 2 is a oxidizing agent (i.e. it gained electrons).
 Mg + N = (Mg)3(N)2 is a Synthesis reaction where three moles of Magnesium [Mg] and two moles of Nitrogen [N] combine to form one mole of Magnesium Nitride [(Mg) 3 (N) 2]
Mg + N = (Mg)3(N)2 is a Synthesis reaction where three moles of Magnesium [Mg] and two moles of Nitrogen [N] combine to form one mole of Magnesium Nitride [(Mg) 3 (N) 2]
 Magnesium nitride may be prepared by the direct reaction of the elements as shown 3 mg(s)+n2(g)=Mg3N2(s). For each combination of the ff. combinations of reactants …
Magnesium nitride may be prepared by the direct reaction of the elements as shown 3 mg(s)+n2(g)=Mg3N2(s). For each combination of the ff. combinations of reactants …
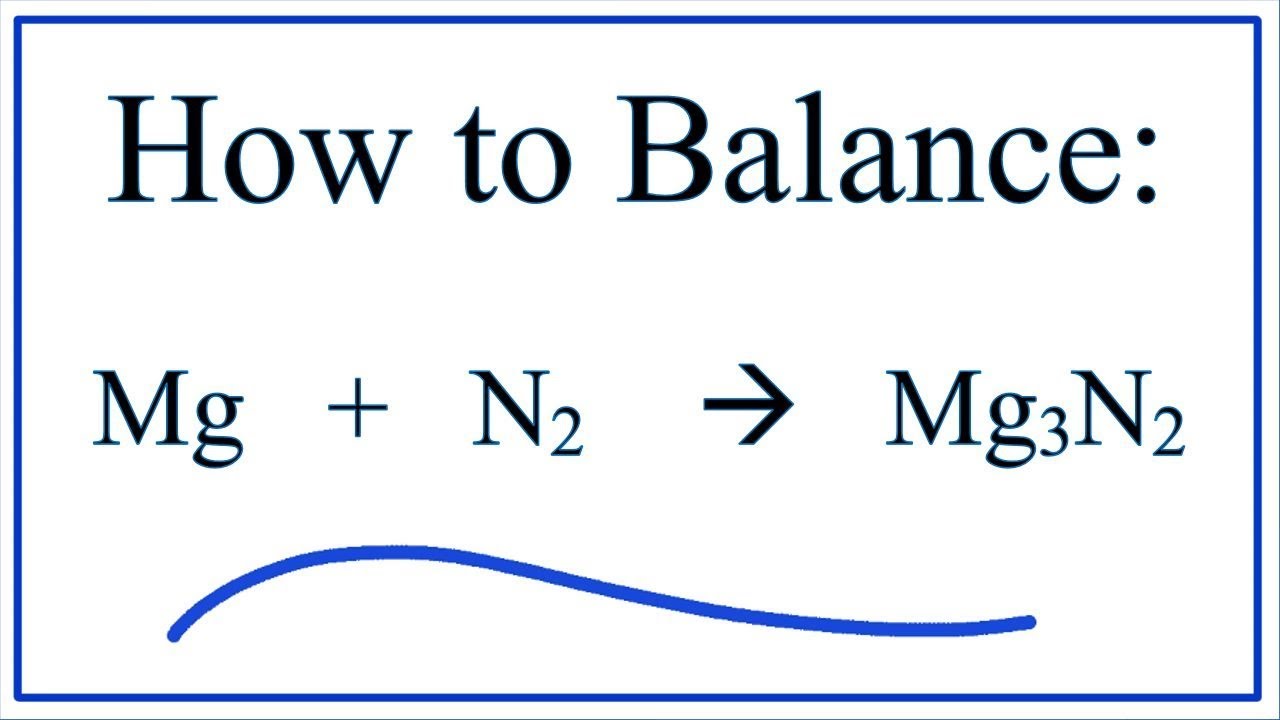
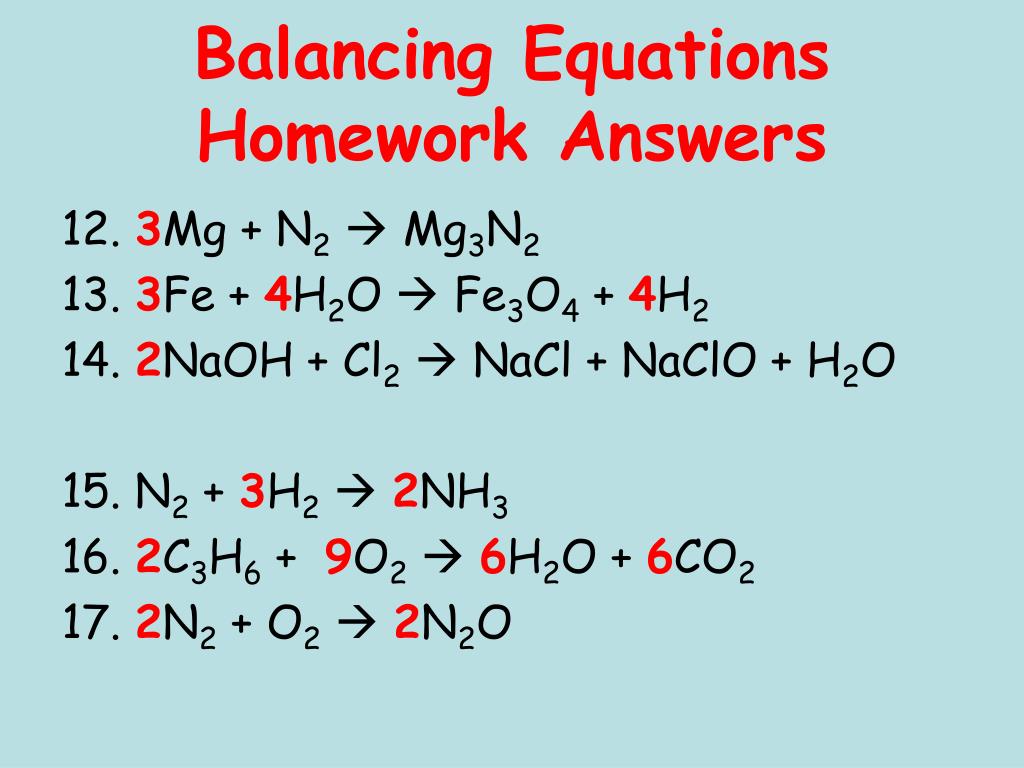 3Mg + N2 → Mg3N2. In this reaction nitrogen acts as oxidising agent. Thus it is reduced, while Mg is reducing agent and it is oxidised because the oxidation number of Mg …
3Mg + N2 → Mg3N2. In this reaction nitrogen acts as oxidising agent. Thus it is reduced, while Mg is reducing agent and it is oxidised because the oxidation number of Mg …
 For the reaction 3 Mg + N2 → Mg3N2 , what is the maximum amount of Mg3N2 which could be formed from 17.32 g of Mg and 0.391 mol of N2? Answer in units of mol
For the reaction 3 Mg + N2 → Mg3N2 , what is the maximum amount of Mg3N2 which could be formed from 17.32 g of Mg and 0.391 mol of N2? Answer in units of mol
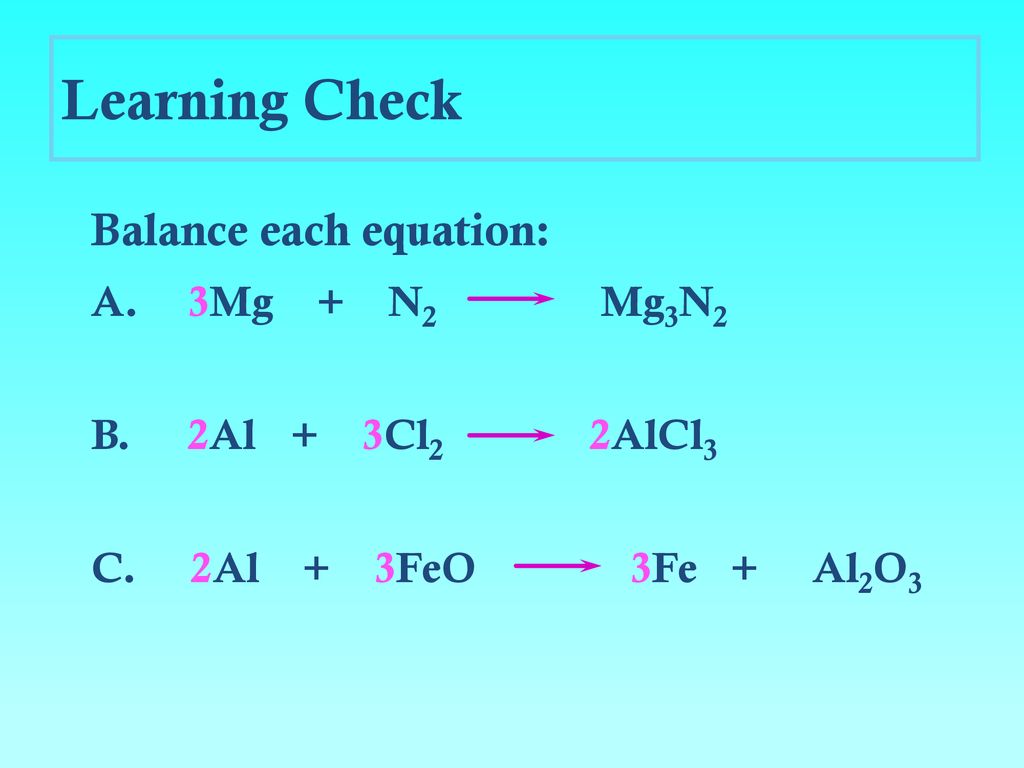
 Is there an error in this question or solution? Balance the following chemical equation.
Is there an error in this question or solution? Balance the following chemical equation.
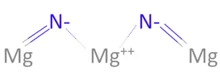
 3 года назад 3Mg + N2 => Mg3N2 Азот окислил магний, в сумме забрал 6 электронов. 3 Mg^0 - 6e 3Mg^-2 2N^0 +6e 2N^-3 Mg - восстановитель N - окислитель
3 года назад 3Mg + N2 => Mg3N2 Азот окислил магний, в сумме забрал 6 электронов. 3 Mg^0 - 6e 3Mg^-2 2N^0 +6e 2N^-3 Mg - восстановитель N - окислитель
 Magnesium nitride, which possesses the chemical formula Mg 3 N 2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder. …
Magnesium nitride, which possesses the chemical formula Mg 3 N 2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder. …
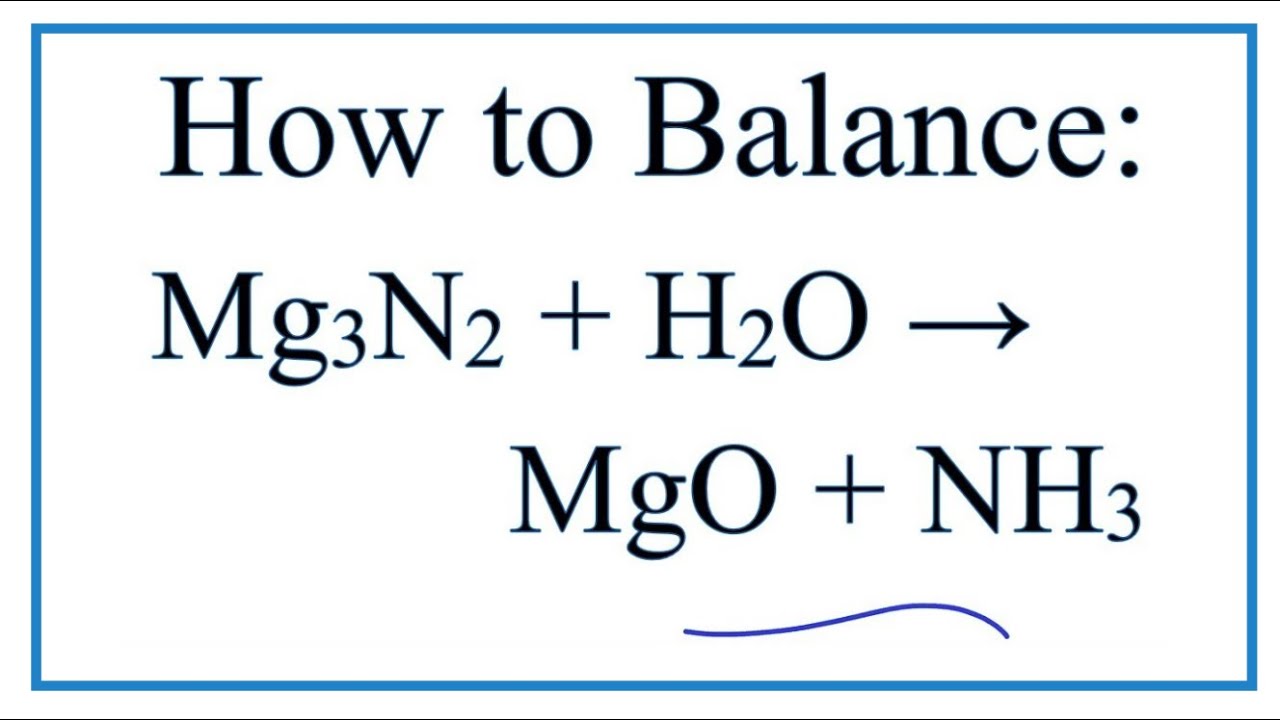
 Это окислительно-восстановительная (редокс) реакция: NH3 является окислителем, Mg является восстановителем. Решенное и коэффициентами уравнение реакции 3 Mg + 2 …
Это окислительно-восстановительная (редокс) реакция: NH3 является окислителем, Mg является восстановителем. Решенное и коэффициентами уравнение реакции 3 Mg + 2 …
 Mg3N2 = Mg + N2 is a Decomposition reaction where one mole of Magnesium Nitride [Mg 3 N 2] decomposes into three moles of Magnesium [Mg] and one mole of Dinitrogen [N 2]
Mg3N2 = Mg + N2 is a Decomposition reaction where one mole of Magnesium Nitride [Mg 3 N 2] decomposes into three moles of Magnesium [Mg] and one mole of Dinitrogen [N 2]
 1 N 2 + 1 Mg = 1 Mg 3 N 2 For each element, we check if the number of atoms is balanced on both sides of the equation. N is balanced: 2 atoms in reagents and 2 atoms in products. Mg is …
1 N 2 + 1 Mg = 1 Mg 3 N 2 For each element, we check if the number of atoms is balanced on both sides of the equation. N is balanced: 2 atoms in reagents and 2 atoms in products. Mg is …
Еще по теме:





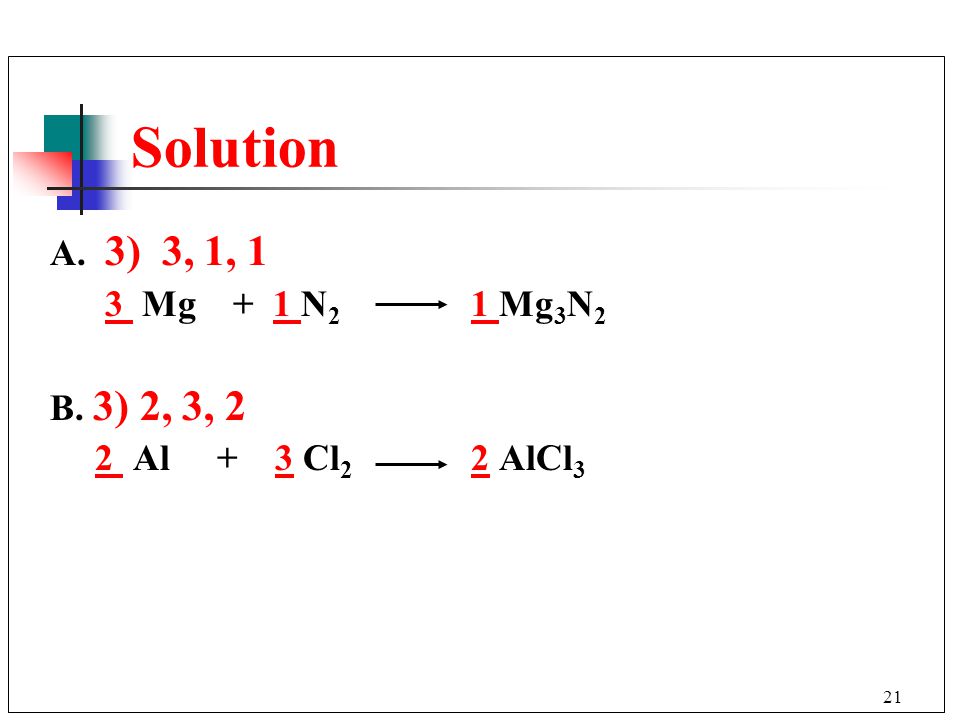
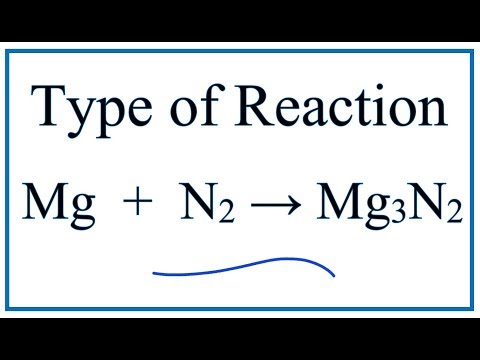






Еще по теме: